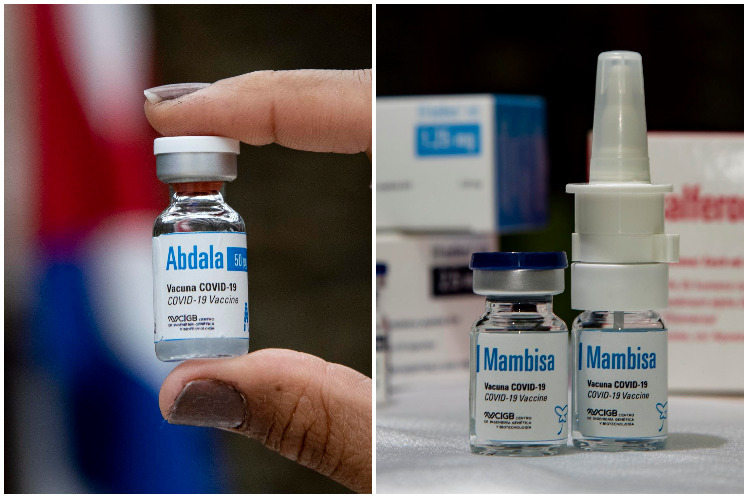
Havana, Jun 18 (RHC)-- A clinical trial aimed at the pediatric population and another one for convalescents are among the new researches with the anti-Covid-19 vaccine candidates Abdala and Mambisa, two of the five developed by Cuban scientists.
Designed by the Center for Genetic Engineering and Biotechnology (CIGB), the study is aimed at children and young people from three to 18 years of age. It will bear the name of Ismaelillo (1882), inspired by the poem dedicated to his son by the Cuban intellectual and independence hero José Martí.
The pediatric research will take place in the easternmost central province of Camagüey, after three months of the start of phase III of the clinical trial with that injectable to more than 48,000 volunteers and its administration in territories and groups at risk due to the deterioration of the epidemiological situation associated with Covid-19.
Pending approval by the regulatory authority, phase I/II with the Abdala vaccine candidate in this population group will be randomized and in parallel groups to evaluate its safety and immunogenicity, its creators explained.
There will be three applications at an interval of 0, 14 and 28 days, but with a lower dose, this time of 25 micrograms, they specified.
The scientific institution will also evaluate convalescents of the disease in its second anti-Covid-19 proposal, designed for nasal administration.
Mambisa, as this formulation is called, will be tested in Phase I/II in four groups of 30 volunteers, three of whom will receive the nasal product and the other will be given the Abdala injectable.

