Cuban medicine Heberprot-P gains space in the US
Havana, May 4 (RHC) The United States Food and Drug Administration has recently granted authorization for the American company Discovery Therapeutics Caribe to carry out phase three clinical trials of the Cuban-made drug Heberprot-P in the North American nation.
According to a note from Cuba’s Biotechnology and Pharmaceutical Industries Business Group, Biocubafarma, this is a phase three protocol for a randomized, double-blind, placebo-controlled trial of the company's leading product.
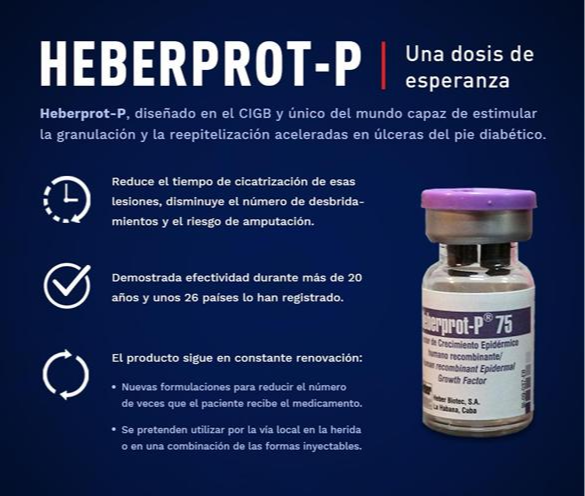
Heberprot-P, created by BioCubaFarma, is the only drug in the world that has reduced the amputation rate in patients with diabetic foot ulcers by up to 75 percent.
It is available in more than 20 countries, but this will be the first time it will be studied in American patients, where it will be proven as an important option in the therapeutic arsenal for the serious condition of the diabetic foot.
In statements to the press at the beginning of this year, the general director of the CIGB, Marta Ayala, pointed out that more than 400 thousand patients with diabetic foot ulcers have benefited from this medication since its initial marketing authorization, issued by the regulatory entity of the Republic of Cuba in June 2006.
She commented that research with other formulations, including nanotechnology, will allow Heberprot-P to be applied in other complex ulcers such as bedsores and venous ulcers generated by vascular disorders.
Dr. David Armstrong, distinguished podiatric surgeon of the University of Southern California and world-renowned diabetic foot ulcer researcher, emphasized the critical need for innovative therapies.
It is estimated that more than 37 million Americans live with diabetes and up to 34 percent of these will suffer from diabetic foot ulcers. Some 154,000 patients with such injuries that cannot heal undergo amputations.
Added to this, almost half of patients undergoing amputation of lower extremities related to diabetic foot ulcers do not survive more than five years, as explained by Dr. Charles Zelen, an expert on this topic.
A report from the Mexican newspaper La Jornada indicated that Discovery Therapeutics Caribe, a biotechnology company that carries out testing of the new drug, was founded specifically to obtain authorization from the federal government in order to market the Cuban treatment in the United States.
The company has a research and collaboration agreement with the CIGB in Havana and an exclusive agreement to market Heberprot-P in the US market and other countries. (Source: PL)

