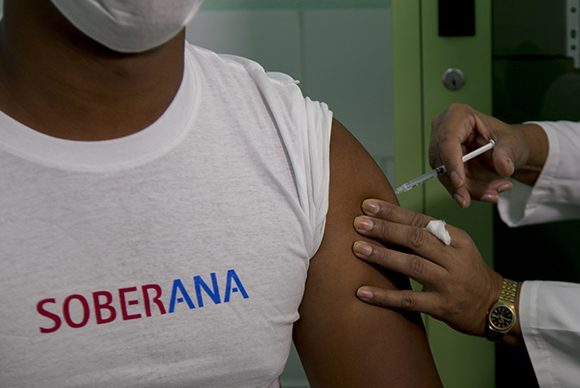
Havana, January 18 (RHC)-- The Cuban COVID-19 vaccine candidate Soberana 02, developed by the Finlay Institute of Vaccines, began on Monday the second stage of phase two of the clinical trials in the municipality of Plaza de la Revolución, in Havana.
Some 405 volunteers received the first dose at a local health facility of the municipality. At the same time, more than 100 already were vaccinated at another clinic at tLa Lisa municipality, west in the Cuban capital.
Last November 2, the first stage of Soberana 02's phase one started with the inclusion of 40 volunteers, and the preliminary results of the study demonstrated the effectiveness of the drug.
Once this phase two concludes, the candidate should proceed to phase three to involve some 150 thousand volunteers.

