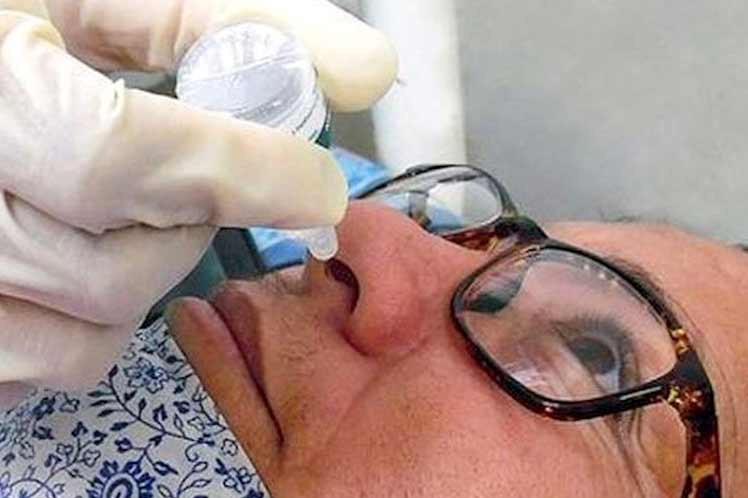
Havana, April 14 (RHC)-- Cuba's nasal vaccine candidate against Covid-19, called Mambisa, is currently on the list of the five vaccines of its type created in the world and is considered safe.
Marta Ayala, director of the Center for Genetic Engineering and Biotechnology (CIGB), explained that Mambisa is the only one of its kind based on the technological platform of recombinantly produced antigens.
Among its main advantages is safety, the adverse effects recorded are slight, and the possibility of administering multiple doses to reinforce the immune response over time, the expert pointed out.
Another benefit, said Ayala, lies in its potential to induce this type of action in the nasopharyngeal mucosa, which is particularly convenient since it is a vaccine candidate against a disease whose entry point is through the respiratory tract.
Ayala said one of the proteins that form part of this product is the nucleocépside or protein that makes up the virus's nucleus.
It has the capacity to stimulate the immune response, combining it with the RBD protein, said the head of the CIGB, quoted by Granma newspaper.
In this regard, the director of Biomedical Research at CIGB, Gerardo Guillén, explained that Mambisa culminated the Phase I clinical study in 88 volunteers, carried out at the National Toxicology Center.
It demonstrated a good safety level and the preliminary results also revealed an effective immunological action, said Guillén.
In studies carried out with people who had suffered from the disease, Mambisa proved to be a very good candidate to reinforce the immune system by administering a single dose, even six months after the infection occurred, Guillén said.
Considering the simplicity of its use and high safety, Mambisa could serve as a booster dose for immunization schemes with other vaccines that, due to their nature or the adverse reactions they cause, cannot be used in multiple applications, the specialist commented.
Guillén added that a Phase I/II clinical study is currently being coordinated in people previously infected with the virus, who will receive a single dose of the vaccine candidate, to evaluate the safety and capacity of this single administration of Mambisa to boost immunity.

