Washington, October 18 (RHC)-- Cuba's Soberana 02 anti-Covid-19 vaccine was recognized by the prestigious U.S. medical journal Annual Review of Medicine, a step towards its possible international homologation.
In an article by opinion leaders, the authors considered that the rapid development and deployment of mRNA vaccines and adenovirus vectors against the disease continues to astound the global scientific community.
However, these drug platforms and production approaches have yet to achieve global equity for the anti-Covid-19 vaccine, cautioned Peter J. Hotez and Maria Elena Bottazzi, inventors of the Corbevax technology, manufactured in India under the ownership of Baylor College of Medicine, Texas, Houston.
Immunizing the billions of people at risk of contracting the pandemic-causing SARS-CoV-2 virus in low- and middle-income countries still depends on the availability of vaccines produced and scaled up through traditional technological approaches, they reflected.
Whole inactivated virus (WIV) and protein-based vaccines offer enormous promise for filling gaps in access to the anti-Covid-19 biologic, especially for the world's low- and middle-income nations in Africa, Latin America, and Southeast Asia, they warned.
An attractive feature of these is their processing capacity in low- and middle-income countries, especially in Brazil, Cuba, Mexico, South Africa, China, India, Indonesia, and elsewhere, they said.
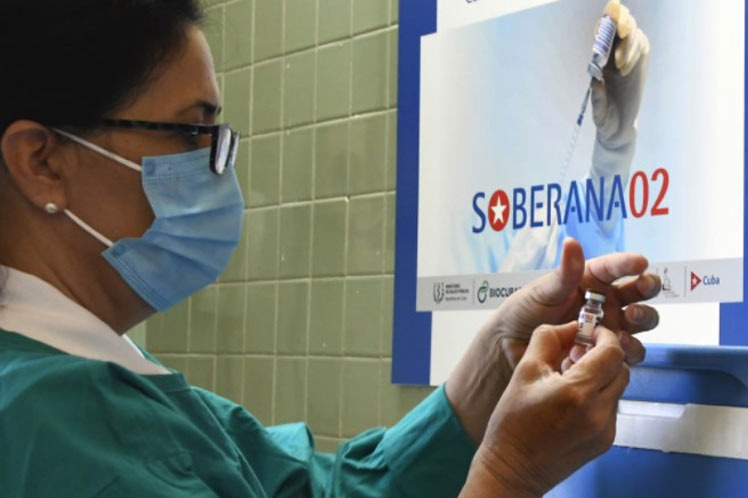
Regarding the Cuban Soberana 02, from the Finlay Vaccine Institute, they pointed out that it is also an RBD candidate, but it is expressed in mammalian cells (CHO, it is immunogenic in mice, as well as being authorized for emergency use in the Caribbean island, where it is also approved for children over two years of age.
In addition, they pointed out, it was authorized for emergency use in Iran (a State that knows it as PasteurCovac, in collaboration with the Pasteur Institute).
Overall, they stressed, these vaccines show excellent safety profiles and, in some cases, demonstrated their potential to induce high levels of virus-neutralizing antibodies and T-cell responses (and protection) both in non-human primates and in preliminary human studies.

